Biomarkers for Diagnosis of Ear and Sinus Infections
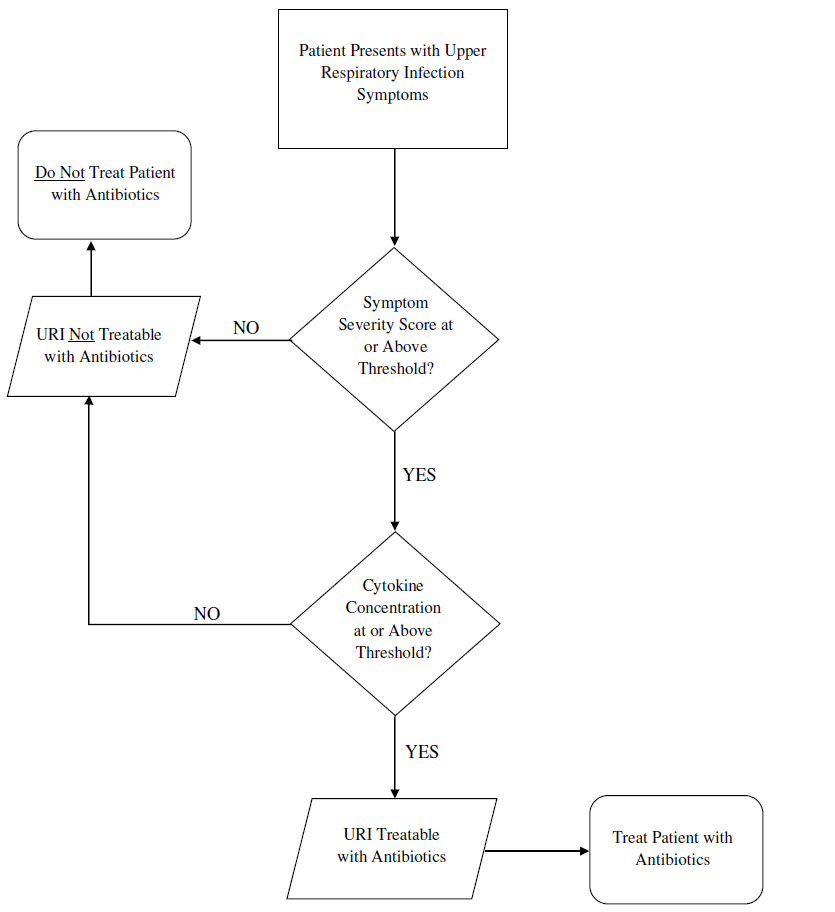
A University of Pittsburgh researcher has identified a novel biomarker, nasal interleukin-1 alpha (IL-1a) for the diagnosis of ear and sinus infections. It can be used with an algorithm that combines IL-1a concentrations with symptom scores from the Pediatric Rhinosinusitis Symptom Scale (PRSS) or Acute Otitis Media–Severity of Symptoms (AOM-SOS), to diagnose ear and sinus bacterial infections in children more accurately. Such a clinical decision support tool could dramatically improve the diagnosis and treatment of patients, reducing harm from antibiotic overtreatment.
Description
Bacterial sinusitis diagnosis results in approximately 8 million antibiotic prescriptions for children in the US each year. However, research suggests that many children may have a viral infection for which antibiotics provide no therapeutic benefit. Unnecessary treatment with antibiotics can result in nausea, headaches, or diarrhea, and contributes to the growing public health challenge of antimicrobial resistance. Identifying only those patients who would benefit therapeutically from antibiotics is key to reducing overprescribing and maintaining good antimicrobial stewardship. This novel diagnostic tool will ensure that only patients with an active bacterial sinusitis infection receive antimicrobial therapy.Applications
• Bacterial sinusitis infection• Antimicrobial stewardship
Advantages
The symptoms of bacterial sinusitis, other respiratory tract infections, and allergies are common and non-specific. The diagnostic criteria for bacterial sinusitis rely solely on the duration and quality of symptoms, leading to regular misdiagnoses of bacterial sinusitis and subsequent unnecessary treatment with antibiotics.This novel diagnostic tool is designed for children between 2 and 12 years of age and would address the clinical need for reliable bacterial sinusitis diagnosis. Levels of IL-1a can be determined from nasal swabs or secretions and analyzed using an ELISA assay. Comparison of IL-1a levels to validated symptoms scores (e.g., PRSS) assists in the identification of patients most likely to benefit from antibiotic treatment. Further development of this method could lead to the production of lateral-flow-assay strips to allow testing for IL-1a levels above a given threshold in primary care or remote settings, allowing for rapid diagnosis and removing the need for laboratory expertise.
Invention Readiness
A multi-center, randomized, double-blind, placebo-controlled parallel group trial identified threshold combinations of IL-1a and PRSS scores in a subset of children who most benefited from antibiotic treatment. Application of these thresholds in a decision-making process could identify children with bacterial sinusitis.IP Status
Patent PendingRelated Publication(s)
Conway, S. J., Mueller, G. D., & Shaikh, N. (2024). Antibiotics for Acute Sinusitis in Children: A Meta-Analysis. Pediatrics, 153(5), e2023064244. https://doi.org/10.1542/peds.2023-064244
Shaikh, N., Hoberman, A., Shope, T. R., Jeong, J. H., Kurs-Lasky, M., Martin, J. M., Bhatnagar, S., Muniz, G. B., Block, S. L., Andrasko, M., Lee, M. C., Rajakumar, K., & Wald, E. R. (2023). Identifying Children Likely to Benefit From Antibiotics for Acute Sinusitis: A Randomized Clinical Trial. JAMA, 330(4), 349–358. https://doi.org/10.1001/jama.2023.10854
