Hibernation-Inspired Protein Reverses Fatty Liver Disease and Obesity
Leveraging cross-species insights from hibernation biology, University of Pittsburgh scientists, in collaboration with colleagues at the University of Michigan and Wayne State University, have developed an siRNA-based therapy that silences an aberrantly reactivated fetal liver enzyme to reverse fatty liver disease and obesity.
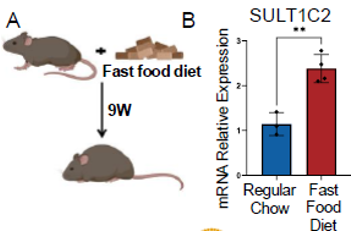
Description
Inspired by hibernation biology, researchers have developed a novel therapy for fatty liver disease and obesity. Mammalian hibernation offers a natural blueprint for metabolic flexibility; hibernators survive months of cold and food scarcity by slowing their heart rate, lowering body temperature, and shifting from glucose to fat as their primary energy source. Drawing on these insights, the team identified molecular pathways relevant to human metabolic disease. Metabolic dysfunction-associated steatohepatitis (MASH), a severe form of fatty liver disease affecting nearly one-quarter of the global population, is marked by excessive fat buildup and disrupted metabolic homeostasis. Current therapies are limited and primarily focus on symptom management. Through comparative analysis of hibernating squirrel livers, the research team identified a fetal liver enzyme, SULT1C2, that is aberrantly reactivated in MASH. In MASH human hepatocytes, siRNA silencing of SULT1C2 eliminated lipid buildup, while liver-targeted siRNA delivery with lipid nanoparticles (LNPs) in a preclinical mouse model reversed obesity and insulin resistance. This innovation restores lipid metabolism through liver-specific gene silencing, offering a disease-modifying approach for one of the world’s most prevalent metabolic disorders.Applications
• Treatment of MASH• Gene-targeted intervention for insulin resistance and metabolic syndrome
• Therapeutic management of obesity-related liver dysfunction
Advantages
Unlike current treatments, which often yield limited results or carry side effects, this siRNA-LNP therapy offers a precise, liver-targeted solution. It silences SULT1C2, a fetal liver enzyme reactivated in MASH, and promotes the liver’s ability to burn fat. In preclinical models, this approach significantly reduced liver fat, improved insulin sensitivity, and reversed obesity without liver toxicity. By focusing on a root-cause gene target using a clinically validated delivery system, the therapy offers disease-modifying potential with fewer systemic risks. Its dual benefit for both fatty liver disease and obesity uniquely positions it in a growing, underserved therapeutic market.Invention Readiness
The therapy has demonstrated strong efficacy in human MASH hepatocytes and mouse models using siRNA-LNP delivery. In vitro, fat accumulation was fully cleared in diseased cells; in vivo, mice showed reduced liver fat, weight loss, and improved glucose control. No toxicity was observed. The invention is at a preclinical stage with established proof-of-concept and a clear path toward translational development.IP Status
Patent PendingRelated Publication(s)
Faccioli, L. A. P., Sun, Y., Animasahun, O., Motomura, T., Sasaki, K., Takebe, T., & Vallier, L. (2025). Human–induced pluripotent stem cell–based hepatic modelling of lipid metabolism associated with the TM6SF2 E167K variant. Hepatology, 82(3), 512–524. https://doi.org/10.1097/HEP.0000000000001065
Rizvi F., Zur, G., Gao, H., Prohaska, A., Wang, J., Venkataraman, D., … (2023). VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion and liver regeneration. Stem Cell Reports, 20(9). https://doi.org/10.1016/j.stemcr.2023.08.022
Collin de l’Hortet, A., Takeishi, K., Guzman-Lepe, J., Morita, K., Achreja, A., Popovic, B., Wang, Y., Handa, K., Mittal, A., Meurs, N., Zhu, Z., Weinberg, F., Salomon, M., Fox, I. J., Deng, C-X., Nagrath, D., & Soto-Gutierrez, A. (2019). Generation of human fatty livers using custom-engineered induced pluripotent stem cells with modifiable SIRT1 metabolism. Cell Metabolism, 30(2), 385–401.e9. https://doi.org/10.1016/j.cmet.2019.06.017
