Innovative Screening Device for Early Detection of Retinal Ischemia in Diabetics
University of Pittsburgh researchers have developed a novel screening device for detecting retinal ischemia in diabetics. This device aims to address the limitations of current screening methods by providing a quantitative, objective measure of retinal ischemia using a non-invasive, non-mydriatic approach. The device could revolutionize the screening process for diabetic retinopathy, making it more efficient and accessible in primary care settings.
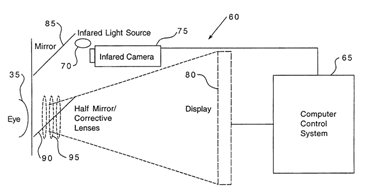
Description
The screening device uses pupillography to measure the eye’s response to light, providing a quantitative assessment of retinal ischemia. It features a dual light source: one that illuminates the central retina and another that illuminates the midperipheral retina. The device measures the pupil’s response to these light sources, with a built-in computer controlling the light sources and recording the responses. The ratio of the light intensities provides a measure of midperipheral retinal sensitivity, indicating the presence of ischemia.Applications
- Screening of diabetics in primary care settings for diabetic retinopathy- Prospective treatment trials to prevent proliferative diabetic retinopathy
- Screening patients with sickle-cell retinopathy
