NextGenET
Each year, over 70 million patients worldwide require placement of a tube in the throat to assist in breathing during surgery or due to disease or emergency. Of these intubated patients, 20 million will require mechanical ventilation. While intubation is a life-saving procedure for patients who cannot breathe on their own, the risk of contracting ventilator-associated pneumonia (VAP) is high at 15% and one in three patients with VAP will die. VAP can also result in additional hospital costs of up to $50,000, and costs the US healthcare system $7.5 billion annually.
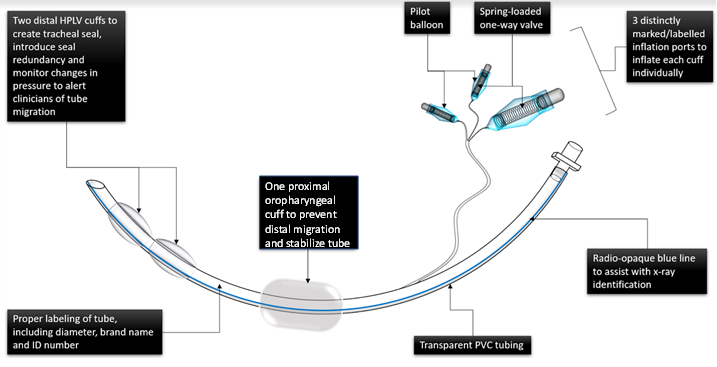
Description
NextGenET is the first endotracheal tube that both prevents ventilator-associated pneumonia and is economically suitable for widespread deployment across an entire healthcare system. Our radical redesign of the current standard endotracheal tube includes a cuff-less design to eliminate cuff-related shortcomings and minimize production costs. Current cuff design allows longitudinal folds to form, which create channels for microaspirations, resulting the accidental aspiration of reflux material in 20-40% of intubated patients. Current cuffs also require constant monitoring to ensure adequate inflation and avoid excess pressure. NextGenET decreases VAP incidence by 50%, avoids cuff-related complications and malfunction, decreases need for monitoring, and prevents tube migration. Compared to standard endotracheal tubes, NextGenET is also competitively priced.Applications
· Hospital ICUs· Intubating patients during surgery
· Ambulances and emergency services
· Ambulatory surgical centers
Advantages
· Limits gastric aspiration and microaspirations· Limits tube movement for patient safety and comfort
· Avoids cuff-related complications
· Decreases the need for monitoring
· Competitively priced compared to standard tubes
