Novel Architecture for Dendrite Free Li-ion Batteries
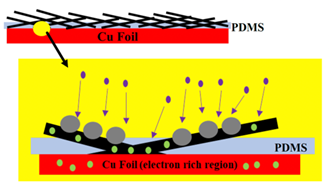
University of Pittsburgh scientists have developed a novel electrode to produce dendrite-free, high-efficiency lithium-ion batteries (LIBs) together with a fabrication method to produce these electrodes. Using electrospun carbon nanofibers (CNF), a polydimethylsiloxane (PDMS) layer on copper foil can produce an electrode (CNF-PDMS/Cu) suitable for stabilization of lithium metal plating for subsequent use in LIBs. This novel CNF-PDMS/Cu architecture has a high columbic efficiency (CE) and is stable over hundreds of cycles. These novel electrodes could produce dendrite-free Li-metal anodes and lead to the development of high-performance, low-cost LIBs revolutionizing the LIB market.

Description
LIBs are used in many consumer goods including electric vehicles, laptops, and medical devices with demand for these batteries growing. The global market for LIBs could exceed $400bn in the next five years to match consumer need for portable power. A major roadblock in meeting the growing demand for LIBs is low CE and the formation of lithium dendrites on the electrodes during plating (i.e., deposition of Li on the anode surface), leading to battery failure and safety risks, including fires. This novel approach aims to improve the capacity of LIBs through increasing CE and reducing the impacts of lithium plating on capacity or safety of the LIB. Using a copper foil and Li-impervious polymer (PDMS), dendrite-free Li-metal anodes can be produced with high specific capacity (>2000 mAh/g) and CE (>99.2%).Applications
• Lithium-ion battery development• Consumer electronics
• Electric vehicles
