Novel Treatment for Neuroblastoma and Ewing Sarcoma
University of Pittsburgh and Children’s Hospital of Philadelphia researchers have developed a panel of fully human antibody binders to the cell surface receptor GDNF family receptor alpha 2 (GFRA2). These neurotrophic factors are highly expressed in neuroblastoma (NB) tumors with a correlation between GFRA2 expression and poor prognosis and could be a novel immunotherapeutic target for precision therapy. The development of antibodies that can specifically bind to GFRA2 opens the door to novel targeted therapies including antibody drug conjugates (ADCs), chimeric antigen receptor (CAR T), and bispecific antibody therapy to treat patients with NB or Ewing sarcoma (ES).
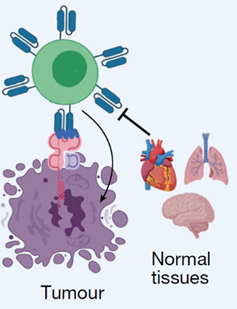
Description
GFRA2 is present on the surface of cells and a receptor for neurturin (NRTN). Binding of NRTN to GFRA2 initiates a downstream signaling cascade promoting neuroblastoma cell survival and proliferation. In addition to increased GFRA2 expression in NB cells, recent research also found GFRA2 highly expressed in ES cells. The development of antibodies to specifically target GFRA2 could be a novel treatment approach in these serious pediatric cancers. Targeting of cancer cells using uniquely or highly expressed surface proteins, not or minimally expressed in health cells using antibodies, can lead to less toxic therapies including CAR T cell and ADC-based approaches. This targeted approach can reduce off-target effects in patients while treating the cancer, improving patient outcomes.Applications
• Neuroblastoma• Ewing sarcoma
Advantages
Immunotherapy, harnessing the body’s own immune system to target cancer, has revolutionized cancer treatment. While immune checkpoint inhibitor therapy is beneficial in treating some cancers, it remains ineffective in many childhood cancers, likely due to the low mutational burden of these cancers.Given the pediatric cancers NB and ES both express GFRA2 which is important to tumorigenesis, GFRA2 is a novel therapeutic target. These antibodies selectively target NB and ES cells through binding to GFRA2 and can be uniquely engineered into different modalities (e.g., monoclonal antibodies, bispecific antibodies, ADCs, or CAR T) and tailored to the disease being treated. These antibodies are fully human or highly humanized reducing the risk of an immunogenic reaction.
Invention Readiness
Using a single domain VH and Fab antibody phage display library, a full panel of fully human antibody binders to GFRA2 have been developed. In vitro experiments have confirmed these binders selectively target recombinant GFRA2 protein and not the homologues GFRA1 and A2. Binding to neuroblastoma cells overexpressing GFRA2 in a concentration-dependent manner was confirmed by flow cytometry.IP Status
Patent PendingRelated Publication(s)
Yarmarkovich, M., Marshall, Q.F., Warrington, J.M., Rasika Premaratne, Farrel, A., Groff, D., Li, W., Moreno di Marco, Runbeck, E., Truong, H., Toor, J.S., Tripathi, S., Nguyen, S., Shen, H., Noel, T., Church, N.L., Weiner, A., Kendsersky, N., Martinez, D. and Weisberg, R. (2023). Targeting of intracellular oncoproteins with peptide-centric CARs. Nature, 623(7988), pp.820–827. https://doi.org/10.1038/s41586-023-06706-0.
