Small Molecule Drug for Targeting Resistant Prostate Cancer
University of Pittsburgh researchers have developed a new class of small molecules that selectively degrade androgen receptor (AR) protein inside the nucleus, offering a novel treatment strategy for castration-resistant prostate cancer (CRPC).
Description
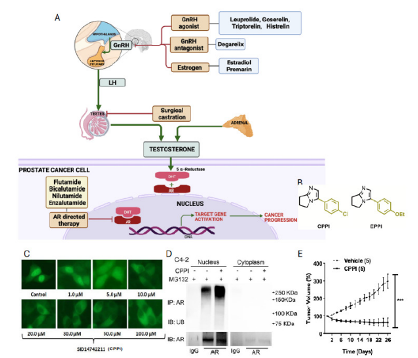
Prostate cancer (PC) is the most diagnosed cancer in men and the second leading cause of male cancer deaths in the U.S. The androgen receptor (AR) plays a central role in PC progression, particularly in castration-resistant prostate cancer (CRPC), where AR remains active despite androgen deprivation therapy. Current anti-androgen therapies are often undermined by resistance, commonly via nuclear AR signaling. Researchers identified that CPPI and EPPI compounds could block AR activity. Building on this discovery, the team developed novel CPPI analogs that directly bind to AR, promoting its degradation within the nucleus. These analogs represent a new class of nuclear AR degraders (NARDs), capable of reducing tumor growth even in therapy-resistant models.Applications
• Castration-resistant prostate cancer treatment.• Enzalutamide-resistant prostate tumor therapy.
• Potential to treat AR-driven cancers beyond prostate cancer.
Advantages
Current prostate cancer therapies target androgen receptor (AR) activity but often fail in advanced stages due to persistent AR signaling and nuclear localization. The CPPI-derived compounds developed at the University of Pittsburgh offer a novel mechanism: they enter the nucleus, bind directly to AR, and trigger its degradation. This approach bypasses common resistance pathways and provides on-target specificity for AR-positive cancer cells, with minimal impact on AR-negative cells. The lead compounds show potent anti-proliferative activity (IC50 <1 µM) and work in animal models of relapsed cancer. These drug-like molecules are synthetically accessible and provide a powerful platform for treating resistant CRPC.Invention Readiness
The lead compounds have been synthesized and tested in vitro and in vivo with validated mechanisms of action. The structure-activity relationship (SAR) has been characterized across 50+ analogs, and key lead compounds show efficacy in the animal model.IP Status
Patent PendingRelated Publication(s)
Yang, Z., Wang, D., Johnson, J. K., Pascal, L. E., Takubo, K., Avula, R., Chakka, A. B., Zhou, J., Chen, W., Zhong, M., Song, Q., Ding, H., Wu, Z., Chandran, U. R., Maskrey, T. S., Nelson, J. B., Wipf, P., & Wang, Z. (2020). A novel small molecule targets androgen receptor and its splice variants in castration-resistant prostate cancer. Molecular Cancer Therapeutics, 19(1), 75-88. https://doi.org/10.1158/1535-7163.MCT-19-0489
Cole, R. N., Chen, W., Pascal, L. E., Nelson, J. B., Wipf, P., & Wang, Z. (2022). (+)-JJ-74-138 is a novel noncompetitive androgen receptor antagonist. Molecular Cancer Therapeutics, 21(4), 483–492. https://doi.org/10.1158/1535-7163.MCT-21-0432
Lv, S., Song, Q., Chen, G., Cheng, E., Chen, W., Cole, R., Wu, Z., Pascal, L. E., Wang, K., Wipf, P., Nelson, J. B., Wei, Q., Huang, W., & Wang, Z. (2021). Regulation and targeting of androgen receptor nuclear localization in castration-resistant prostate cancer. Journal of Clinical Investigation, 131(4), e141335. https://doi.org/10.1172/JCI141335
