

A Novel Universal Pre-Targeting Strategy for Molecular Imaging and Radionuclide Therapy
One of the major barriers toward the wide use of radiolabeled monoclonal antibodies (mAb) for molecular imaging and radionuclide therapy are the relatively high uptakes in non-tumor organs. Pre-targeting strategies present a promising approach to achieve improved sensitivity and specificity in small animal models, however, there are still a couple of limitations preventing them from being widely accepted for clinical use, including: 1) they are restricted to non-internalizing antibody, because the antibodies internalized into tumor cells are no longer available for ligation with RM; and 2) they have low tumor uptake, which consequently requires greater amounts of radionuclide to achieve high quality imaging and effective radiotherapy.In order to overcome these limitations, we have developed a novel universal pre-targeting strategy that substantially improves the sensitivity, specificity, and tumor uptake of radiotracer while with the broader scope of applicable mAbs (both internalizing and noninternalizing ones), thereby advancing current pre-targeting methods and facilitating their application within the biomedical sciences.
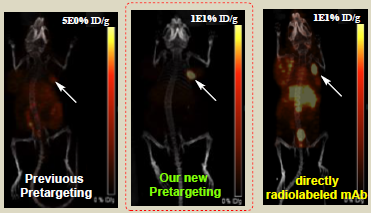
Description
In the previous pre-targeting strategies, the administrated radionuclide (with no tumor-targeting ligand) only provides low tumor uptake, which can be attributed to: 1) the administrated radionuclide itself has very low concentration on tumor; and 2) it is washed away from tumor very fast. The present invention relates to a dual-receptor pre-targeted (DRPT) molecular imaging and/or targeted drug delivery method, wherein an imaging label and/or therapeutic drug is integrated into a tumor-targeting small molecule that carries a moiety and also binds to the pre-administered tumor-specific antibody. Therefore, this small molecule, binding to both the pre-administered tumor-specific antibody and a biomarker on tumor, can be continuously trapped on tumor due to the generation of a slowclearing bioconjugate (ligation product between the small molecule and the preadministered tumor-specific antibody), and consequently resulting in increased sensitivity, increased specificity, improved signal to noise ratio, and greater applicability, as both internalizing and non-internalizing probes (e.g., antibodies) can be used.Applications
Molecular ImagingRadionuclide therapy
Biochemistry/Molecular Biology
Biomedical Sciences
Advantages
Increased sensitivity and specificity of molecular imaging agentsImproved tumor uptake Exceptional signal-to-noise ratio
Broadened scope of usable mAbs