

Personalized Assembloids as AMD Models
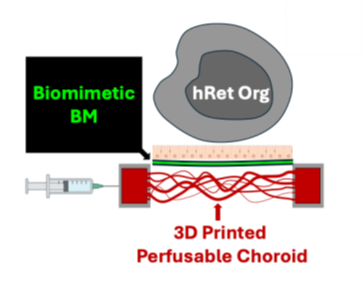
University of Pittsburgh researchers are developing a novel assembloid platform to model age-related macular degeneration (AMD). This platform, produced from cells derived directly from the patient, comprises of photoreceptors (PR), retinal pigment epithelium (RPE) cells and a novel biomimetic of Bruch’s membrane (BM) perfused on top of a donor-specific 3D printed choroidal vasculature. This first donor-derived model allows for in vitro study of the interactions between each of these layers and improved understanding of the pathology of AMD. This platform could aid the diagnosis and understanding of AMD in addition to predicting disease progression and the development of new therapies.
Description
AMD affects approximately 200 million people globally and is one of the leading causes of vision loss in later life. Atrophic AMD, where cells in the macula are damaged, accounts for half of all serious cases. To date, no treatment exists for atrophic AMD and with increasing prevalence of the disease there is an urgent clinical need to develop treatment strategies. BM is a multi-layered structure in the eye between the choroid and RPE. Consisting of extracellular matrix (ECM), BM plays a vital role in the transport of oxygen, electrolytes and nutrients, and the development of AMD. This novel donor-derived platform can replicate interactions and synergies between BM, PR, RPE and other tissues, and could revolutionize AMD research.Applications
• Age-related macular degeneration (AMD) research• Drug development
Advantages
The absence of a suitable research model for AMD has limited the understanding of the disease and the development of successful therapies. Rodent models lack similarity to the human eye and non-human primates are costly, also lacking an appropriate AMD model. In vitro 2D models fail to accurately replicate the complexity of the eye or consider the role of choroidal vasculature and BM. This novel 3D model overcomes these shortcomings. Using a patient’s blood, hiPSCs are used to generate human retinal organoids (hRetOrg), RPE cells (hiRPE) and choroidal endothelial cells (CECs). A novel biomimetic multilayered BM produced by electrospinning of ECM components has also been developed. The novel hRetOrg-hiRPE-BM assembloid is constructed and perfused on a 3D printed perfusable choroidal vasculature specific to the patient’s anatomy derived from in vivo imaging and allows for understanding of the role of choroidal vasculature on RPE health and function.Invention Readiness
A proof-of-concept assembloid has been produced. Immunostaining data have confirmed physical contact and potential functional interactions between the hRetOrg and hiRPE-BM. Further development and optimization efforts are on-going.IP Status
Patent PendingRelated Publication(s)
Ong, J., Selvam, A., Driban, M., Zarnegar, A., Morgado Mendes Antunes Da Silva, S. I., Joy, J., Rossi, E. A., Vande Geest, J. P., Sahel, J.-A., & Chhablani, J. (2025). Characterizing Bruch’s membrane: State-of-the-art imaging, computational segmentation, and biologic models in retinal disease and health. Progress in Retinal and Eye Research, 106, 101358. https://doi.org/10.1016/j.preteyeres.2025.101358