

Thermoresponsive Gel for Cysteamine Delivery
University of Pittsburgh and New York University scientists have developed a thermoresponsive eye gel drop for ocular delivery of cysteamine. This novel approach could act as a “weekly eye drop” and could significantly improve the lives of patients with cystinosis by removing the need for hourly use of invasive eye treatments.
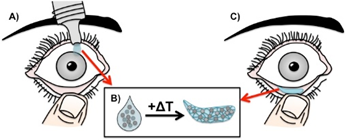
Description
Cystinosis is a rare metabolic, recessive genetic disease leading to accumulation of cystine crystals. In the eye, these crystals accumulate in the cornea causing severe impact on vision. Current therapeutic approaches are highly invasive with eye drops requiring administration every waking hour to dissolve developing crystals. This novel approach, building on a previous innovation (Pitt ID 3683), uses a thermoresponsive hydrogel incorporating cysteamine microspheres (CMS) which can be administered as a liquid to the eye which forms a gel in the fornix at body temperature. This gel will slowly release medication over a sustained period through hydrolysis of the microspheres, removing the need for hourly eyedrops and improving the quality of life of patients.Applications
• Corneal cystinosis• Ocular administration of medication
Advantages
Current treatment for corneal cystinosis is Cystaran eye drops (cysteamine ophthalmic solution). While effective, these drops need to be administered every waking hour, between 6–12 times a day. The drops can be extremely irritating for patients and can only be stored in a refrigerator for around one week. For this chronic condition, drops are administered over a patient’s lifetime, causing thousands of irritations each year and severely impacting on quality of life.Using this novel thermoresponsive gel, it is possible to dramatically reduce the number of administrations required. A single drop could deliver up to a week’s supply of cysteamine. Additionally, this new formulation significantly increases the stability, allowing for storage of over 25 days, improving access to medication and reducing waste.
Invention Readiness
Using a previously developed gel, CMS were incorporated, and gel formation was found to occur at 33.9˚C. In vitro drug release kinetics found cysteamine was released over 24 hours from this formulation, in therapeutically useful dosages. CMS extended the stability of cysteamine to 7 weeks at 4˚C and 5 weeks at 25˚C. Animal studies found drops could be easily administered, leading to gel formation with no signs of irritation. It was found the gel could deliver cysteamine at dosages equivalent to 12 drops over a 24-hour period. Large animal and human studies are required.IP Status
https://patents.google.com/patent/US20210369649A1Related Publication(s)
Jimenez, J., Resnick, J. L., Chaudhry, A. B., Gertsman, I., Nischal, K. K., & DiLeo, M. V. (2022). Ocular biodistribution of cysteamine delivered by a sustained release microsphere/thermoresponsive gel eyedrop. International Journal of Pharmaceutics, 624, 121992. https://doi.org/10.1016/j.ijpharm.2022.121992
Jimenez, J., Washington, M. A., Resnick, J. L., Nischal, K. K., & Fedorchak, M. V. (2021). A sustained release cysteamine microsphere/thermoresponsive gel eyedrop for corneal cystinosis improves drug stability. Drug Delivery and Translational Research, 11(5), 2224–2238. https://doi.org/10.1007/s13346-020-00890-6
Bruk, L. A., Dunkelberger, K. E., Khampang, P., Hong, W., Sadagopan, S., Alper, C. M., & Fedorchak, M. V. (2020). Controlled release of ciprofloxacin and ceftriaxone from a single ototopical administration of antibiotic-loaded polymer microspheres and thermoresponsive gel. PLOS ONE, 15(10), e0240535. https://doi.org/10.1371/journal.pone.0240535