

Novel Small-Diameter Vascular Graft for Tissue Engineering
University of Pittsburgh researchers have developed a novel three-layered, bio-inspired, small-diameter tissue engineered vascular graft (TEVG). The three distinct layers are readily produced by electrospinning (ES), molding of decellularized cardiac tissue gel (cardiac extracellular matrix [cECM]), and thermal induced phase separation (TIPS). These TEVGs mimic the structure and function of native coronary arteries (CA) and could revolutionize access to CA grafts.
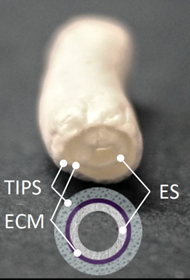
A three-layered small-diameter vascular graft has been produced that can mimic the function and structure of native coronary arteries. It is a promising solution to the lack of suitable vessels readily available for use in CABG surgery. The three layers are produced by electrospinning (ES), thermal induced phase separation (TIPS) and molding of decellularized cardiac tissue (cECM) gel.
Description
Coronary Heart Disease (CHD), when blood vessels in the heart muscle become narrow or blocked, is currently the most common cause of death in developed nations. In the US alone, one person dies from CHD every 85 seconds. Successful treatment requires rapidly restoring blood flow to affected areas of the heart through medical or surgical interventions. In the most severe cases, the gold standard treatment is a coronary artery bypass graft (CABG), where autologous vessels (e.g., saphenous vein, radial artery or gastroepiploic artery) are used to bypass the blocked coronary artery. Limitations on tissue availability make this treatment suboptimal and inaccessible for many patients. There remains an urgent clinical need to develop alternative grafts to bypass coronary blockages. TEVGs have the potential to improve the availability of suitable grafts for coronary artery bypass surgery, improving outcomes for millions of patients globally.Applications
• Coronary heart disease• Coronary artery bypass graft surgery
• Other vascular graft surgery
Advantages
The lack of suitable blood vessels due to problems such as donor-site morbidity, limit access to CABG for many patients. Some alternative graft options have been developed. However, artificial polymer graft patients require daily anticoagulation therapy which can induce mechanical mismatch at the graft-tissue interface. Biological tissue grafts have also been developed and while superior to artificial grafts, problems with control of the structure and function of the graft along with failure linked to thrombogenicity and intima hyperplasia remain frequent.This novel TEVG combines the benefits of synthetic and biological tissue-derived grafts. Using ES, cECM, and TIPs production methods, grafts are built with three layers designed to mimic different functions of native grafts (e.g., intima, media, adventitia). These TEVGs mimic native grafts on the macro-, meso-, and microscopic tissue level. Additionally, the production method allows for improved control of the structure and function of grafts along with industrial scalability
Invention Readiness
Prototype TEVGs have been produced with an inner diameter of 2 mm and an outer diameter of 3 mm with a wall thickness of 0.5 mm. Synthetic polymeric layers have been combined with a bioactive layer to promote tissue formation and mitigate intima hyperplasia. In vitro studies confirmed that these TEVGs presented comparable structure and function to native CA.IP Status
https://patents.google.com/patent/US20240216587A1Related Publication(s)
Luketich, S. K., Cosentino, F., Di Giuseppe, M., Menallo, G., Nasello, G., Livreri, P., Wagner, W. R., & D’Amore, A. (2022). Engineering in-plane mechanics of electrospun polyurethane scaffolds for cardiovascular tissue applications. Journal of the Mechanical Behavior of Biomedical Materials, 128, 105126. https://doi.org/10.1016/j.jmbbm.2022.105126
D’Amore, A., Luketich, S. K., Raffa, G. M., Olia, S., Menallo, G., Mazzola, A., D’Accardi, F., Grunberg, T., Gu, X., Pilato, M., Kameneva, M. V., Badhwar, V., & Wagner, W. R. (2018). Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. Biomaterials, 150, 25–37. https://doi.org/10.1016/j.biomaterials.2017.10.011